FAQ
FAQs about Zaiput separators
What can Zaiput separators be used for?
Zaiput separators separate any process fluid containing 2 or more immiscible phases. This includes oil/water, aqueous/organic, organic/organic, gas/liquid and more. The Zaiput separators can be inserted into any continuous or batch process for inline or downstream separation.
What is the pressure rating of the Zaiput separators?
Thanks to their special design with a metal shell, Zaiput separators can be operated at a high pressure, up to 290 psi/2 MPa. However, it is important to ensure that the pressures of the two outlets are not considerably different. Consult our technical support team for special use at high pressure.
Two phases have very similar densities. Can Zaiput separators separate them?
Separation in the Zaiput separator is an interfacial phenomena, not gravity-based. Therefore, the Zaiput separators can be used with any fluid mixture, regardless of their density difference.
Why is the pressure control important for successful membrane operation?
Successful membrane separation requires an optimal pressure difference across the two sides of the membrane, i.e. retained and permeate sides. Zaiput separators incorporate an on-board pressure controller such that users no longer need to keep regulating the pressures of the two sides of the membrane. Click here to learn more about how it works.
What makes the Zaiput separators unique among conventional extractors?
Zaiput separators provide instantaneous, complete phase separation. They require neither a coalescing plate nor a settling tank. Their unique design eliminates the need for electronic apparatus for agitating parts or online process control. Additionally, they are very easy-to-use, inexpensive, and perfectly designed for a wide range of chemical environments (e.g., organic solvents, acidic solutions, high pressure).
Can Zaiput separators handle acidic/basic solutions?
All of the wetted parts inside the Zaiput separators are highly chemical-compatible. The metal shell does not contact the fluid. Zaiput separators can handle most organic solvents as well as corrosive solutions.
Do Zaiput separators perform differently if I heat or cool them?
Temperature affects interfacial phenomena. Heating tends to decrease the interfacial tension of liquid mixtures. At very high temperatures, the liquid mixture is close to the miscibility region and may be difficult to separate. Contact us to consult our technical support team for special use with extreme temperatures.
What throughput can the Zaiput separators accommodate?
Currently, we provide Zaiput separation devices in three versions, SEP-10, SEP-200 and SEP-3000. These versions cover flows ranging from 0 to 3000 mL/min.
Is the Zaiput separation device the same thing as a cross-flow filtration unit?
The Zaiput separation devices separate immiscible fluids based on their wettability. This is different from filtration which uses a size-exclusion technique. Filtration generally removes solid from one liquid phase (either a pure liquid or a miscible liquid mixture).
What are the recommended chemical processes to use with the Zaiput separator?
Zaiput’s separators can be used for any type of chemical process, in either the laboratory or in an industrial setting. They are suitable for high-value chemicals thanks to their very small dead volume and high efficiency. Below is a glimpse of potential applications:
- Inline workup for synthesis (organic/inorganic)
- Separation of biphasic mixtures
- Homogeneous catalyst recovery
- Liquid-liquid extraction
- Liquid-gas separation
- Recovery of pharmaceuticals or fine chemical compounds
- Separation of alcohols from organic solvents
- Removal of excess starting materials
- Countercurrent extraction
How often should I change a membrane after use with chemicals?
The membranes used in the Zaiput separators are highly compatible with chemicals so it can function for weeks. However, depending on how clean the separated fluid is, the membrane may need to be replaced more frequently. This is especially true if the fluid contains a significant amount of solid particles/precipitates.
For any more questions or further information, please contact us.
FAQs about the Zaiput back pressure regulators (BPRs)
Can Zaiput back pressure regulators (BPRs) handle multiphasic fluids?
Zaiput BPRs work well with multiphasic fluids such as liquid/liquid and gas/liquid. They are also capable of handling small amounts of solid particles with diameters smaller than 150-200 μl.
How precise are the Zaiput BPRs?
The set point of the Zaiput BPRs are adjustable by compressed air/gas. The Zaiput BPR have been designed to maintain a stable pressure with negligible variation (± 5%).
How do I connect a Zaiput BPR into my process?
The inlet and outlet of the Zaiput BPR-10 and BPR-1000 are listed in the specifications tab.
How often do I have to reset the Zaiput BPR?
Zaiput BPRs are designed to be leak-proof. They have been characterized to be able to maintain a stable pressure for at least 10 days.
What are the differences between a back pressure regulator and a pressure regulator?
A back pressure regulator is used to regulate inlet pressure, so an upstream fluid will be pressurized to the set point. On the other hand, a pressure regulator regulates outlet pressure, so a downstream fluid will be pressurized to the set point.
What makes the Zaiput BPRs unique?
Unlike many other products on the market, Zaiput BPRs do not come with a factory-preset pressure. Instead, users are allowed to precisely set the Zaiput BPR to any pressure within a recommended operating range (1 – 290 psi). Zaiput BPRs are also easy-to-use and chemically compatible.
Are the Zaiput BPRs acid-resistant?
All of the wetted parts inside the Zaiput BPR are made of highly chemical-compatible materials. The metal shell will not be in contact with the fluid. They can handle most organic solvents as well as acidic solutions. Contact our technical support team for questions about chemical compatibility.
What are the minimum and maximum flow rates that can be operated by the Zaiput BPR?
The recommended operating flow rate is between 0 and 1000 mL/min, depending on the unit.
Can the Zaiput BPR be heated for some solid handling applications?
The internal parts of Zaiput BPRs are made of polymers. Therefore, the recommended operating temperature is below 130 ˚C. For any application at higher temperatures, consult our service team for a customized solution.
General FAQ
What is flow chemistry?
In flow chemistry, chemical reactions occur in a flowing fluid rather
than a stirred tank (batch). This generally requires pumps and a reactor
that can be monitored for specific temperatures and pressures. This type
of production is developed to replace the batch production as well as to
facilitate continuous manufacturing.
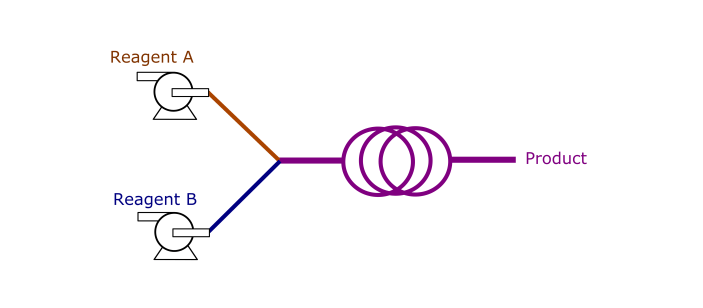
What are advantages of flow chemistry over batch chemistry?
-
Safer
In batch, reagents are loaded into a reactor at
once. A large volume of hazardous chemicals can be unsafe. This is
opposed to flow chemistry, where reagents are gradually and
continuously sent to a reactor. The smaller volumes provides a safe
environment to carry out aggressive reactions. Also, headspace is
absent in flow reactions, and thereby the reactor can be easily
pressurized by a back pressure regulator (BPR). -
More efficient
With smaller sizes, flow reactors promote
heat and mass transfers by providing a high surface area to volume
ratio. This increases overall kinetics of the reactions. For some
processes, multiple reactions need to be operated in series. In flow,
they can be telescoped so that unstable intermediates can be sent to
another flow reactor before chemical decomposition. -
Better control
Residence time can be precisely controlled
by matching a reactor’s volume with a flow rate. Also, with better
heat transfer, the reactor can be maintained uniformly at a specified
temperature. Heat can dissipate out effectively from the reactor in
case of exothermic reactions. -
Easier to scale
Fluid hydrodynamics (e.g. mixing) is much
simpler in flow than in batch. Agitation in batch typically generates
non-uniform droplet sizes, which are difficult to predict across
different scales.
What are known examples of chemical syntheses that are done in flow?
There have been many chemical syntheses that benefit from flow, such as
highly exothermic reactions that require efficient cooling and
photochemistry that require a large surface upon exposure to light.
Multistep synthesis containing sensitive metal-hydride or potentially
explosive organic azides can be telescoped in a closed flow system such
that those chemical species can be consumed as soon as they are
generated from the previous step.
- Reduction with metal hydride (e.g. DIBALH reduction)
- Exothermic oxidation
- Organic azide chemistry, click reaction
- Photochemistry
- Solid-supported synthesis
- Organometallic chemistry (e.g. Grignard reaction)
- Nanoparticle synthesis
- Coupling reaction
What are different modes of membrane separation?
A membrane or a semi-permeable material is commonly used for separation
because it consumes less energy than such other techniques as
distillation and crystallization. Almost all industries, ranging from
food to pharmaceutical to biotechnology, use membrane technology in at
least one of their unit operations. The separation in a membrane can be
driven by pressure or by a concentration gradient. The membrane can be
fabricated from organic material (e.g., polymers), or inorganic
materials (e.g., ceramic, zeolite, emulsion liquid).
Fundamentally, the applications of membranes can be divided into three
major categories:
Particle exclusion by pore size
This approach uses a membrane to physically capture particles or
molecules that are larger than the pore sizes or the molecular-weight cut
off of membranes. The examples of this technique include microfiltration,
ultrafiltration, and nanofiltration.
Diffusion facilitating barrier
Components are first absorbed into a membrane. The separation factor
depends on the difference in solubility and diffusion among different
components. The examples using this type of approach include gas
permeation, pervaporation, and supported-liquid membrane.

Membrane extraction
In this application, a membrane acts as a barrier for two immiscible
phases. One of the phases will be preferentially wetting the membrane
while the other is non-wetting. Each phase carries a certain amount of
species of interest.

What is liquid-liquid extraction?
Liquid-liquid extraction refers to contact between two immiscible liquid
phases. One of the phases is a feed containing components to be
separated while the other is generally a solvent. The latter sometimes
contains an extractant, a chemical species that facilitate extraction by
binding or forming molecular complexes with components of interest.
The two liquid phases that come out of the extractor are called the
raffinate and the extract. The raffinate refers to the phase rich in the
same carrier solvent as the feed, while the extract refers to the phase
rich in the extraction solvent. One example is the extraction of
succinic acid from water using ethyl acetate: the feed is the aqueous
solution of succinic acid, the extraction solvent is ethyl acetate, the
raffinate is the remaining aqueous solution of succinic acid, and the
extract is the succinic acid in ethyl acetate.
How to quantify performance of extraction?
We can assess whether the chosen solvent is suitable by examining the
partition ratio. The partition ratio (K), or interchangeably termed as
partition coefficient or distribution ratio, is defined as the ratio of
the solute concentration in the extract phase to that in the raffinate
phase after one equilibrium extraction. The partition ratio is
dimensionless, but it is important to note that concentration units used
in the calculation of K as different units could produce different
values of K.
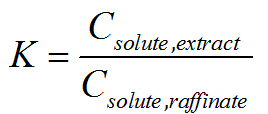
Sometimes, the extraction involves more than one component. A separation
factor, αi, j, is used to assess how selectively the extraction can
separate different components. The separation factor, αi, j, is
typically defined as the partition ratio of component i divided by that
of component j.
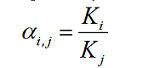
What are major types of extraction?
Extraction can be classified by different criteria:
|
|
|
|
|
|
|
|
|
Standard extraction is aimed to extract one solute from the feed to the
solvent while fractional extraction is aimed to separate two or more
components such that a fraction of components are leaving with the
raffinate and others are leaving with the extract phase. The analogies
of standard and fractional extractions are gas stripping and fractional
distillation, respectively.
Extraction can be set up to be performed multiple times to increase
separation levels. The extraction can be operated with single-stage,
crosscurrent, and countercurrent. The crosscurrent extraction involves
sequential extractions. The raffinate from a previous stage will be in
contact with the fresh solvent. The countercurrent extraction requires
an arrangement such that the feed and the extraction solvent have
countercurrent (i.e., opposite) flows.
What liquid-liquid extraction equipment is available?
Selection of extractors can vary from one industry to another. Many
decisions depend on feed and solvent properties such as density
difference, viscosity, stability of solute, and interfacial tensions.
Sometimes, the extractor is chosen because of the industry’s experience
and knowledge of the equipment and scale-up procedure.
Simplified drawings show major types of liquid-liquid extraction
equipment: (far left) static column, (second from left) agitating
column, (top right) mixer settler, (bottom right) membrane technology.
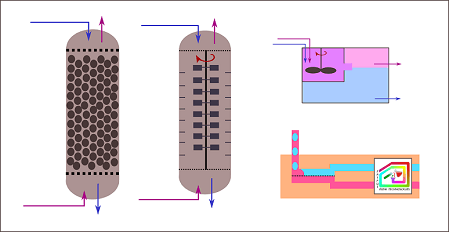
Static extraction column
This type of equipment refers to a column containing trays or
packing materials. They are often used in petrochemical industries. The
application is limited to systems with low viscosity, low to moderate
interfacial tension, and only a few number of stages. The disadvantages
are low mass-transfer efficiency due to the absence of active agitation.
Agitated extraction column
For systems with moderate to high interfacial tensions, agitation
may be needed to create dispersion with better control of drop size and
distribution. Examples are rotating-impeller, Scheibel, and
reciprocating-plate columns. The scale-up procedure involves rough
hydrodynamic and geometric similarity estimation between pilot-plant and
production-scale designs. Occasionally, individual stages are structured
differently to accommodate variation across the column.
Mixer-settler
Mixer settlers are similar to batch-wise separatory funnels. They
are commonly used in hydrometallurgical and high-value chemical
productions because of its flexibility and ability to handle solids. It
generally provides high stage efficiency, as the mixing speed and settling
time can be precisely controlled. Nevertheless, the excessively strong
mixing is problematic because it creates small droplets that are difficult
to separate by settling.
Centrifugal extractors
In this type of extractors, centrifugal force is used to
simultaneously facilitate the mass transfer and liquid-liquid phase
separation. This equipment is particularly useful for systems with small
density difference or with unstable solutes that require short contact
time. However, the centrifugal machine is expensive, and requires high
maintenance costs.
Membrane-based extractors
Membrane-based extractors separate two liquid-liquid phases using
selective wettability. So, it is more suitable for systems containing
liquids with similar densities. It can be done for a wide range of
flowrates. The separation is completed within short time and with minimal
energy consumption.
What are typical challenges in operating extractors?
Axial mixing
This refers to the behavior of the dispersed and continuous phase
flows that deviate from an ideal plug flow. This increases a residence
time distribution and minimizes the solute concentration driving force
throughout the equipment. Therefore, the overall performance is decreased.
To resolve the problem, the equipment is designed to accommodate a larger
capacity or to guide the direction of the liquid phase.
Flooding
This phenomenon refers to a breakthrough of one liquid phase to an
outlet of the other liquid phase in a countercurrent flow operation.
Flooding often occurs because of excessive solvent-to-feed ratio,
throughput and agitation intensity. A plot between a certain process
variable and the maximum throughput before the flooding begins to occur is
called a flooding curve. Many columns are operated at 40-60 % of the
flooding point because the actual process may contain surface-active
impurities and variations in the design.
Short residence time required
Many systems contain unstable solutes that require short contact
time. Gravity-based columns and mixer-settlers are not suitable because of
the slow coalescence. Centrifugal and membrane-based extractors allow high
mass-transfer and phase-separation rates.
Emulsions
Stable dispersion cause problems like flooding. Formation of
emulsions can be promoted by low interfacial tension and density
differences and the presence of surfactants. According to Perry’s
handbook, systems with < 3 mN/m or Δρ < 0.05 are likely to form
stable dispersions, which take several hours or longer to collapse.
Reference: Perry, Robert H., D. W. Green, and J. O. Maloney. “Perry’s
handbook of chemical engineering.” Perry’s Handbook of Chemical
Engineering (1997).

