
Zaiput Flow Technologies’ back pressure regulators are specifically designed for the needs of the flow chemist. Our devices feature a unique combination of high accuracy and precision, broad pressure range and outstanding chemical compatibility in a compact format.
The back pressure regulator compares the pressure of the fluid to be controlled (main stream) to a reference pressure. Flow of the main stream is allowed only if its pressure meets the reference pressure.
In addition, our pressure regulators have the unique feature that the set point is selected by the user. The user selects the back pressure set point by pressurizing air inside a dedicated chamber of the device. The set point is equal to the pressure of the air inside the device.

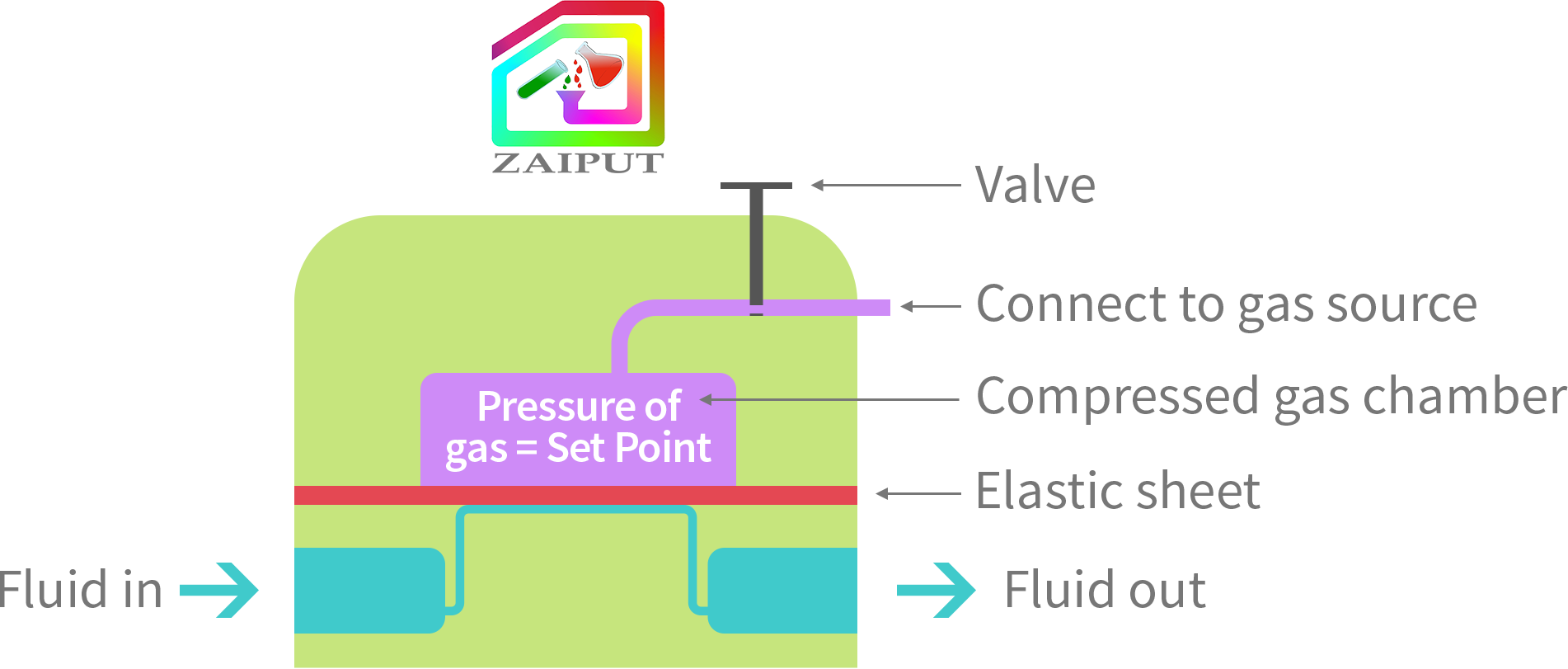
Zaiput’s BPRs have a chamber that the user fills with compressed air. The pressure of the inputted air represents the back pressure set point of the device. An elastic sheet intercepts the process fluid flow path and allows a comparison of the pressure of the process fluid to the reference pressure. In other words, the process fluid can flow through the device only when its pressure is equal to the reference pressure.
Zaiput’s BPRs are carefully designed with chemically compatible materials as well as a reliable dynamic response of an elastic sheet. With a sealed valve, the BPRs can be used in two modes:
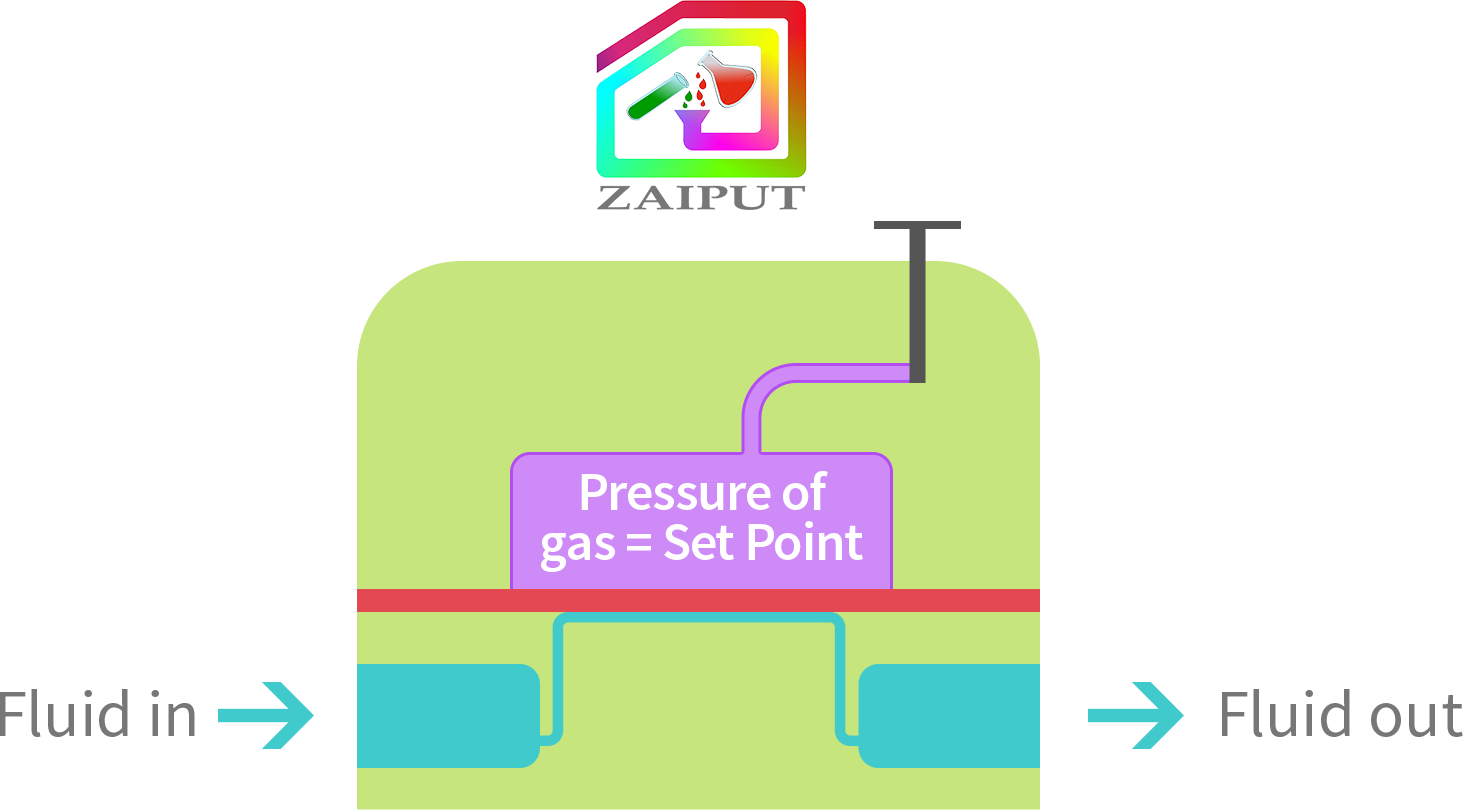
In this mode, the user compresses gas into the chamber of the device at any certain pressure P. Then, the user manually closes the valve; P inside the chamber is now at the set point. The user can disconnect the BPR from the pressure source (e.g., gas tank). If another set point is required, this procedure can be repeated by setting the pressure of the top chamber to the new value. The advantage is that a gas tank is not required for operation.
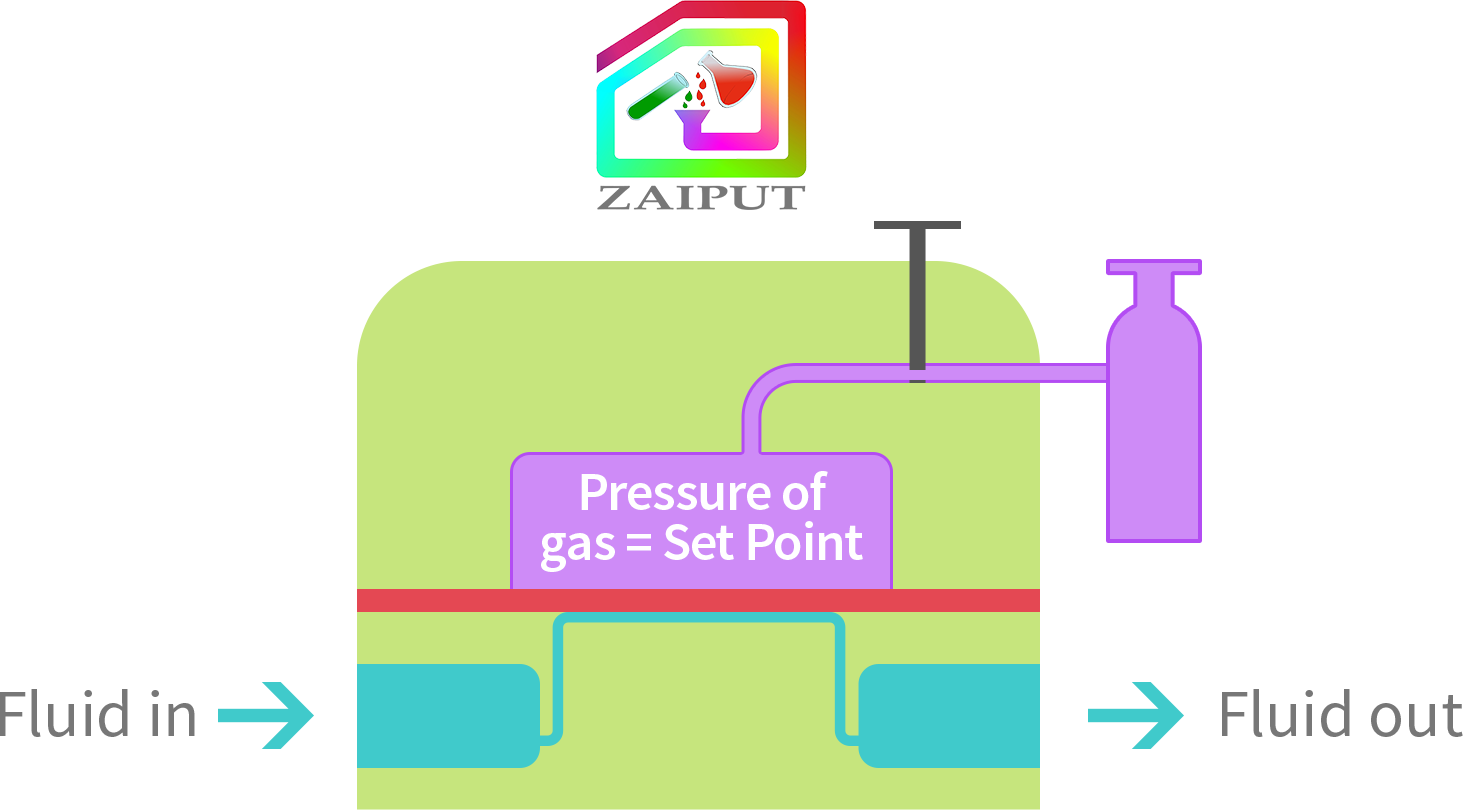
Some processes require the fluid pressure to dynamically change over the course of operation. The Zaiput BPR is able to provide such a continuous pressure setting. To achieve this setting, the user connects the Zaiput BPR to the gas/pressure source. Note that the valve is left open. By changing the pressure of the gas source (e.g., the regulator of the gas tank), the set point or P is dynamically changed.
Note that the Zaiput BPR sets a minimum level of pressure for the main fluid flow. If multiple BPRs are placed in series, the pressure of the fluid flow will be equal to the highest pressure set point (i.e., not the sum of the multiple set points). As a result, the device can also be used as a relief valve. Each BPR comes with a connection tube (the external pressurized gas source is not included).
Federico Florit, Anirudh M. K. Nambiar, Christopher P. Breen, Timothy F. Jamison, and Klavs F. Jensen, Design of dynamic trajectories for efficient and data-rich exploration of flow reaction design spaces, React. Chem. Eng., Sept 2021.
Michaela Wernik, Gellért Sipos, Balázs Buchholcz, Ferenc Darvas, Zoltán Novák, Sándor B. Ötvös, and C. Oliver Kappe, Continuous flow heterogeneous catalytic reductive aminations under aqueous micellar conditions enabled by an oscillatory plug flow reactor, Green Chemistry, July 2021.
Baldassarre Venezia, Luca Panariello, Daniel Biri, Juhun Shin, Spyridon Damilos, Anand N.P. Radhakrishnan, Chris Blackman, Asterios Gavriilidis, Catalytic Teflon AF-2400 membrane reactor with adsorbed ex situ synthesized Pd-based nanoparticles for nitrobenzene hydrogenation, Catalysis Today, Feb 2021.
Victor-Emmanuel H. Kassin, Romain Morodo, Thomas Toupy, Isaline Jacquemin, Kristof Van Hecke, Raphaël Robiette, and Jean-Christophe M. Monbaliu, A modular, low footprint and scalable flow platform for the expedient α-aminohydroxylation of enolizable ketones, Green Chemistry, Feb 2021.
Hoang Khang Bui, Ki Yoon Kim, Hyeonjin Kim, Jae‐Pyoung Ahn, Taekyung Yu, and Tae Seok Seo, Total Integration of the Sample Injection, Microdroplet Reaction, Phase Separation, Real‐Time Optical Detection, and Recovery of Diverse Silver–Gold Bimetallic Nanoalloys in a Continuous Process, Particle., Dec 2020.
Ke Wu, Gaowei Wu, Alexander J. MacRobert, Elaine Allan, Asterios Gavriilidis, and Ivan P. Parkin, Ultra high molecular weight polyethylene with incorporated crystal violet and gold nanoclusters is antimicrobial in low intensity light and in the dark, Mater. Adv.., Nov 2020.
Gi Byoung Hwang, Gaowei Wu, Juhun Shin, Luca Panariello, Victor Sebastian, Kersti Karu, Elaine Allan, Asterios Gavriilidis, and Ivan P. Parkin, Continuous Single-Phase Synthesis of [Au25(Cys)18] Nanoclusters and their Photobactericidal Enhancement, ACS Appl. Mater. Interfaces, Oct 2020.
Elena Herrero-Gomez, Cornelis H. M. van der Loo, Lena Huck, Ana Rioz-Martínez, Nandell F. Keene, Bryan Li, Kees Pouwer, Christophe Allais, Photo-oxidation of Cyclopentadiene Using Continuous Processing: Application to the Synthesis of (1R,4S)-4-Hydroxycyclopent-2-en-1-yl Acetate, Org. Process Res. Dev., April 2020.
Cristian Rosso, Sebastian Gisbertz, Jason D. Williams, Hannes P. L. Gemoets, Wouter Debrouwer, Bartholomäus Pieber and C. Oliver Kappe, An oscillatory plug flow photoreactor facilitates semi-heterogeneous dual nickel/carbon nitride photocatalytic C–N couplings, Reaction Chemistry and Engineering, Feb 2020.
Romaric Gérardy, Julien Estager, Patricia Luis, Damien P. Debeckerd and Jean-Christophe M. Monbaliu, Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions, Catalysis Science & Technology, Jan 2020.
Baldassarre Venezia, Mark Douthwaite, Gaowei Wu, Meenakshisundaram Sankar, Peter Ellis, Graham J.Hutchings, Asterios Gavriilidis, Slurry loop tubular membrane reactor for the catalysed aerobic oxidation of benzyl alcohol, Chemical Engineering Journal, Dec 2019.
He Huang, Gi Byoung Hwang, Gaowei Wu Kersti Karu, Hendrik Du Toit, Han Wu, June Callison, Ivan P. Parkin, Asterios Gavriilidis, Rapid synthesis of [Au25(Cys)18] nanoclusters via carbon monoxide in microfluidic liquid-liquid segmented flow system and their antimicrobial performance, Chemical Engineering Journal, Oct 2019.
Róbert Örkényi1, Continuous Purifications in Multistep Continuous Flow Synthesis of Pharmaceutical Compounds, Poster Presentation, Sep 2019.
Anna Pekkari, Zafer Say, Arturo Susarrey-Arce, Christoph Langhammer, Hanna Härelin, Victor Sebastian, Kasper Moth-Poulsen, Continuous Microfluidic Synthesis of Pd Nanocubes and PdPt Core–Shell Nanoparticles and Their Catalysis of NO2 Reduction, ACS Appl. Mater. Interfaces, Aug 2019.
Alexander Steiner Jason D. Williams Juan A Rincón Oscar de Frutos Carlos Mateos C. Oliver Kappe, Implementing Hydrogen Atom Transfer (HAT) Catalysis for Rapid and Selective Reductive Photoredox Transformations in Continuous Flow, European Journal of Organic Chemistry, July 2019.
C Rosso, JD Williams, G Filippini, M Prato, CO Kappe, Visible-Light-Mediated Iodoperfluoroalkylation of Alkenes in Flow and Its Application to the Synthesis of a Key Fulvestrant Intermediate, Organic Letters, June 2019.
Gaowei Wu, Enhong Cao, Peter Ellis, Achilleas Constantinou, Simon Kuhn, Asterios Gavriilidis, Continuous flow aerobic oxidation of benzyl alcohol on Ru/Al2O3 catalyst in a flat membrane microchannel reactor: An experimental and modelling study, Chemical Engineering Science, Volume 201, 29 June 2019, Pages 386-396.
Conor Waldron, Arun Pankajakshan, Marco Quaglio, Enhong Cao, Federico Galvanin, and Asterios Gavriilidis, An autonomous microreactor platform for the rapid identification of kinetic models, React. Chem. Eng., April 2019.
Victor-Emmanuel Horst Kassin, Romaric Gérardy, Thomas Toupy, Diégo Collin, Elena Salvadeo, François Toussaint, Kristof Van Hecke and Jean-Christophe M Monbaliu Expedient Preparation of Active Pharmaceutical Ingredient Ketamine under Sustainable Continuous Flow Conditions, Green Chemistry, March 2019.
René Lebl, David Cantillo, and C. Oliver Kappe, Continuous generation, in-line quantification and utilization of nitrosyl chloride in photonitrosation reactions, React. Chem. Eng., Jan 2019.
Jason D. Williams, Momoe Nakano, Romaric Gérardy, Juan A. Rincón, Óscar de Frutos, Carlos Mateos, Jean-Christophe M. Monbaliu, and C. Oliver Kappe, Finding the Perfect Match: A Combined Computational and Experimental Study toward Efficient and Scalable Photosensitized [2 + 2] Cycloadditions in Flow, Organic Process Research and Development Jan 2019.
Chengwen Xue, Jiesheng Li, Jin Ping Lee, Ping Zhang and Jie Wu, Continuous amination of aryl/heteroaryl halides using aqueous ammonia in a Teflon AF-2400 tube-in-tube micro-flow reactor, Reaction Chemistry and Engineering November 2018.
Manuel Köckinger, Christopher A. Hone, Bernhard Gutmann, Paul Hanselmann, Michael Bersier, Ana Torvisco, and C. Oliver Kappe, Scalable Continuous Flow Process for the Synthesis of Eflornithine Using Fluoroform as Difluoromethyl Source, Org. Process Res. Dev. November 2018.
Zhiguo Wang, Romaric Gérardy, Guillaume Gauron, Christian Damblon and Jean-Christophe M Monbaliu, Solvent-free organocatalytic preparation of cyclic organic carbonates under scalable continuous flow conditions, Reaction Chemistry & Engineering Oct 2018.
Irini Abdiaj, Clemens R. Horn, and Jesus Alcazar, Scalability of Visible-Light-Induced Nickel Negishi Reactions: A Combination of Flow Photochemistry, Use of Solid Reagents, and In-Line NMR Monitoring, The Journal of Organic Chemistry Oct 2018.
Gaowei Wu, Enhong Cao, Peter Ellis, Achilleas Constantinou, Simon Kuhn, Asterios Gavriilidis, Development of a flat membrane microchannel packed-bed reactor for scalable aerobic oxidation of benzyl alcohol in flow, Chemical Engineering Journal Oct 2018.
Nelly Ntumba Tshibalonza, Romaric Gérardy, Zouheir Alsafra, Gauthier Eppeb and Jean-Christophe M. Monbaliu,
A versatile biobased continuous flow strategy for the production of 3-butene-1,2-diol and vinyl ethylene carbonate from erythritol, Green Chemistry Sep 2018.
Carlos Mendoza Noémie Emmanuel Carlos Paez Laurent Dreesen Jean-Christophe M. Monbaliu, and Benoît Heinrichs, Improving Continuous Flow Singlet Oxygen Photooxygenations with Functionalized Mesoporous Silica Nanoparticles, ChemPhotoChem Aug 2018.
Nathalie Ollivier, Thomas Toupy, Ruben C. Hartkoorn, Rémi Desmet, Jean-Christophe M. Monbaliu & Oleg Melnyk, Accelerated microfluidic native chemical ligation at difficult amino acids toward cyclic peptides, Nature Communications July 2018.
Carlos Mendoza, Noémie Emmanuel, Carlos Alberto Páez, Laurent Dreesen, Jean-Christophe M. Monbaliu, Benoît Heinrichs, Transitioning from conventional batch to microfluidic processes for the efficient singlet oxygen photooxygenation of methionine, J. Photochemistry Dec 2017.
Joshua Britton and Timothy F Jamison, The assembly and use of continuous flow systems for chemical synthesis , Nature Protocols Oct 2017.
Amanda C. Wicker, Frank A. Leibfarth and Timothy F. Jamison, Flow-IEG enables programmable thermodynamic properties in sequence-defined unimolecular macromolecules, Polym. Chem. Sep 2017.
Gaowei Wu, Enhong Cao, Simon Kuhn, and Asterios Gavriilidis, A Novel Approach for Measuring Gas Solubility in Liquids Using a Tube-in-Tube Membrane Contactor, Chem. Eng. Technol. Aug 2017.
Róbert Örkényi, Gyula Beke, Eszter Riethmüller, Zoltán Szakács, János Kóti, Ferenc Faigl, János Éles, and István Greiner, Environment-Friendly Synthesis of Indoline Derivates Using Flow Chemistry Techniques, Eur. J. Org. Chem. Aug 2017.
Paul Watts and Cloudius Sagandira, Synthesis of amines, carbamates and amides via multi-step continuous flow synthesis , Eur. J. Org. Chem. Aug 2017.
Noémie Emmanuel, Carlos Mendoza, Marc Winter, Clemens R. Horn, Alessandra Vizza, Laurent Dreesen, Benoît Heinrichs, and Jean-Christophe M. Monbaliu, Scalable Photocatalytic Oxidation of Methionine under Continuous-Flow Conditions Org. Process Res. Dev. July 2017.
Isabel Ortiz de Solorzano, Martin Prieto, Gracia Mendoza, Teresa Alejo, Silvia Irusta, Manuel Arruebo, and Victor Sebastian, Microfluidic Synthesis and Biological Evaluation of Photothermal Biodegradable Copper Sulphide Nanoparticles ACS Appl. Mater. Interfaces Aug 2016.
Maryam Peer, Nopphon Weeranoppanant, Andrea Adamo, Yanjie Zhang, and Klavs F. Jensen, Biphasic catalytic hydrogen peroxide oxidation of alcohols in flow: Scale up and extraction Org. Process Res. Dev. Aug 2016.
Andrea Adamo, Rachel L. Beingessner, Mohsen Behnam, Jie Chen, Timothy F. Jamison, Klavs F. Jensen, Jean-Christophe M. Monbaliu, Allan S. Myerson, Eve M. Revalor, David R. Snead, Torsten Stelzer, Nopphon Weeranoppanant, Shin Yee Wong, Ping Zhang, On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system Science April 2016.
Achilleas Constantinou, Gaowei Wu, Albert Corredera, Peter Ellis, Donald Bethell, Graham J. Hutchings, Simon Kuhn, and Asterios Gavriilidis, Continuous Heterogeneously Catalyzed Oxidation of Benzyl Alcohol in a Ceramic Membrane Packed-Bed Reactor Organic Process Research & Development December 2015.
Chunhui Dai, David R. Snead, Ping Zhang, and Timothy F. Jamison, Continuous-Flow Syn and Purification of Atropine with Sequential In-Line Separations of Structurally Similar Impurities J. Flow Chem. July 2015.
David Snead, Timothy Jamison, A Three‐Minute Synthesis and Purification of Ibuprofen: Pushing the Limits of Continuous‐Flow Processing. AngewandteChemie, Dec 2014.
 |
||
 |
||
| Part Number | BPR-10 | |
|---|---|---|
| Total Flow Rate | 0.05-20 ml/min (for high accuracy) | |
| Max Set Point Pressure | 2 MPa (290 psi) | |
| Dimensions | 52 x 52 x 44 mm | |
| Max Inlet Pressure | 5 MPa | |
| Max Operating Temp | 90 ℃ | |
| Process Fluid Ports | 1/4″ UNF – 28 flat bottom | |
| Wetted parts | Perfluorinated polymers (ETFA, PFA) | |
| Error | < 1% | |
 |
||
 |
||
| Part Number | BPR-1000 | |
| Total Flow Rate | 20-1000 ml/min (for high accuracy) | |
| Max Set Point Pressure | 2 MPa (290 psi) | |
| Dimensions | 97 x 97 x 64 mm | |
| Max Inlet Pressure | 5 MPa | |
| Max Operating Temp | 90 ℃ | |
| Process Fluid Ports | 1/4″ OD tubing with Swagelok PFA fittings and our compression nut | |
| Wetted parts | Perfluorinated polymers (ETFA, PFA) | |
| Error | < 1% | |
*Scroll left to see more of the table
Zaiput BPRs work well with multiphasic fluids such as liquid/liquid and gas/liquid. They are also capable of handling small amounts of solid particles with diameters smaller than 150-200 μl.
The set point of the Zaiput BPRs are adjustable by compressed air/gas. The Zaiput BPR have been designed to maintain a stable pressure with negligible variation (± 5%).
The inlet and outlet of the Zaiput BPR-10 and BPR-1000 are listed in the specifications tab.
Zaiput BPRs are designed to be leak-proof. They have been characterized to be able to maintain a stable pressure for at least 10 days.
A back pressure regulator is used to regulate inlet pressure, so an upstream fluid will be pressurized to the set point. On the other hand, a pressure regulator regulates outlet pressure, so a downstream fluid will be pressurized to the set point.
Unlike many other products on the market, Zaiput BPRs do not come with a factory-preset pressure. Instead, users are allowed to precisely set the Zaiput BPR to any pressure within a recommended operating range (1 – 290 psi). Zaiput BPRs are also easy-to-use and chemically compatible.
All of the wetted parts inside the Zaiput BPR are made of highly chemical-compatible materials. The metal shell will not be in contact with the fluid. They can handle most organic solvents as well as acidic solutions. Contact our technical support team for questions about chemical compatibility.
The recommended operating flow rate is between 0 and 1000 mL/min, depending on the unit.
The internal parts of Zaiput BPRs are made of polymers. Therefore, the recommended operating temperature is below 130 ˚C. For any application at higher temperatures, consult our service team for a customized solution.